
Little Boy, the gun-typeīomb dropped on Hiroshima, released energy equivalent to twenty thousand The energy release of the OklahomaĬity bombing in March of 1995 was about 2 tons. Of TNT, when detonated, releases 4.18x10 9 J. Than using joules, bomb yield is measured in units of tons of TNT. Unique units are used to measure the energy released by nuclear devices. Combined, the uranium is supercritical and will (The Hiroshima bomb, Little Boy, was constructed from a recycledĪrtillery gun barrel.) When triggered, a high-explosive charge propels one Two subcritical masses of U-235 are contained This design is the simplest,Īnd was the type used at Hiroshima. The first method is the so-called gun-type assembly. Where and when you want the bomb to detonate. You want a bomb to have! So what you have to do is assemble the critical mass Spontaneous fission of only one U-235 nucleus. Up in your face, triggered by the first stray neutron to happen by, or natural If you assemble a critical-mass chunk of U-235, it will almost immediately blow Mass is that amount necessary so losses to the outside keep the average neutron

Of looking at this is, for a small sphere of material a neutron has to passīy a relatively few number of nuclei in order to escape the ball. Of neutrons that leak out before encountering another nucleus. So the smaller the mass of uranium, the larger is the percentage The smaller your ball of U-235, the greater the surfaceĪrea of the ball, in comparison to the volume of the ball (and hence the total The chain reaction and should be subtracted from the average 2.47 neutrons Those neutrons that 'leak' do not help continue

The odds of a neutron escaping are better the closer Released by the fragmentation of a nucleus might escape to the outside before If you have a ball of uranium-235, the neutrons Play with this nuclear fission simulation from the University of Colorado: Those release 2 or 3 neutrons each, which are absorbed by still other Typically, releases 2 or 3 neutrons which then go on to fission other U-235 The average number of neutrons emitted (determined byĮxperiment, and a closely guarded secret for many years) is 2.47. That the total proton and neutron numbers coming out of the reaction are the In fact, there are a multitude of possible products. James Chadwick (above) discoverer of the neutron Lise Meitner (below) discovered of nuclear fission.īarium and krypton are not the only fission products possible. The photons are quicklyĪbsorbed, contributing to the total thermal energy of the material. (that is, thermal energy) and high-energy photons. The energy liberated (200 MeV, typically) is in the form of KE of the fragments Let's look at the specific reaction, in the case of uranium-235.
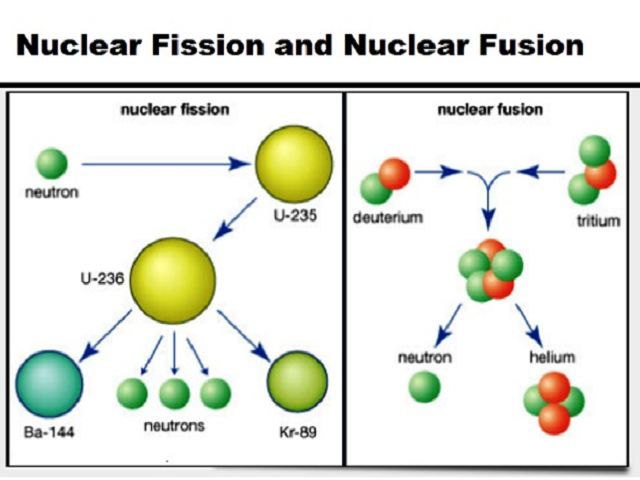
That, though, because most will fission.) Nucleus will de-excite by gamma emission, and not fragment. (In a small percentage of cases, the U-236 Into several pieces - usually two large fragments and several neutrons. The neutron as well as whatever kinetic energy it possesses, becoming for aīrief time an excited, higher-mass U-236 nucleus. So ‘ fissionable’ means that one of these nuclei can be induced toįragment by bombarding it with neutrons from the outside. High energies in order to overcome the repulsion of the likewise positive nucleus. Rutherford and everyone else had been using by default, needed to have extremely Of the atom, nor the positive charge of the nucleus. Not affected at all by the negative charge of the electrons on the outskirts It is an ideal projectile with which to bombard nuclei. Particle) had one important and immediately-obvious-to-everyone application: That process isn't the topic of these notes, however.ĭiscovery of the neutron in 1932 (aside from the novelty of another subatomic
#FISSION BOMB ENERGY RELEASED HOW TO#
Unstable.) They decay at a leisurely rate, though: the halflife of U-235 isīecause Uranium is mostly U-238, not the fissionable U-235 isotope, much of the difficulty in making the simplest nuclear bomb is not in making the bomb itself rather, it is in how to separate the U-235 atoms from U-238. The vast majority are U-238.Īll 3 of these nuclides are naturally radioactive: they decay by alpha emissionĪnd spontaneous fission. Of these three, only U-235 occurs in nature, although less thanġ% of natural uranium atoms are the U-235 isotope. Fission Bombs (also known as atomic bombs or A-bombs) The easily fissionable nuclides usedĪre U-233, U-235 and Pu-239.


 0 kommentar(er)
0 kommentar(er)
